Gene therapy for hemophilia is revolutionizing the way this chronic bleeding disorder is treated, providing hope for countless patients like Terence Blue. Historically, hemophilia treatment required frequent injections of clotting factor, which many found burdensome and anxiety-inducing. However, recent advancements have led to the FDA-approved gene therapy Hemgenix, which aims to create a more permanent solution by providing patients with a functional copy of the gene responsible for producing the missing clotting factor. The benefits of gene therapy extend beyond mere convenience, promising to significantly improve the quality of life for individuals living with hemophilia. As success stories like Blue’s emerge, the potential for gene therapy to transform hemophilia treatment becomes increasingly clear, leading to a future where patients can live without the constant fear of bleeding complications.
In the realm of genetic medicine, innovative therapies are emerging to tackle genetic disorders, particularly hemophilia, a condition marked by uncontrolled bleeding due to clotting factor deficiencies. Recently, groundbreaking alternatives such as Hemgenix have made headlines, showcasing the remarkable impact that gene modification can have on patient care. By introducing corrected genetic material into the body, these therapies offer enduring solutions that could reduce or eliminate the need for ongoing clotting factor treatments. The rise of gene therapy represents a significant advancement in the medical field, with an increasing number of patients sharing their gene therapy success stories, enhancing the narrative around treatment efficacy. As research progresses, it is crucial to examine both the promise and the challenges of these FDA-approved gene therapies to understand their role in future hemophilia treatments.
Understanding Hemophilia: Challenges and Innovations
Hemophilia is a rare genetic disorder that hinders the body’s ability to form blood clots, leading to excessive bleeding even after minor injuries. Historically, this condition has necessitated frequent hospital visits for patients, who must receive infusion treatments of clotting factors to manage their symptoms. For individuals like Terence Blue, managing hemophilia has been an ingrained part of life, filled with routine injections and constant caution to avoid injuries that could lead to severe bleeding. The advent of novel treatments, particularly gene therapy, has prompted a shift in how patients perceive their condition and the management thereof.
Recent developments in hemophilia treatment have introduced advanced options beyond traditional factor replacement therapies. Gene therapy, in particular, has emerged as a groundbreaking approach to address the underlying genetic defects, offering potential long-term benefits. The excitement surrounding therapies like Hemgenix, which targets the specific genetic mutation responsible for hemophilia, indicates a paradigm shift that not only promises to alleviate symptoms but may ultimately lead to a cure. Understanding the complexities of hemophilia treatment will help in navigating the journey toward transforming patients’ lives.
Gene Therapy for Hemophilia: A New Era
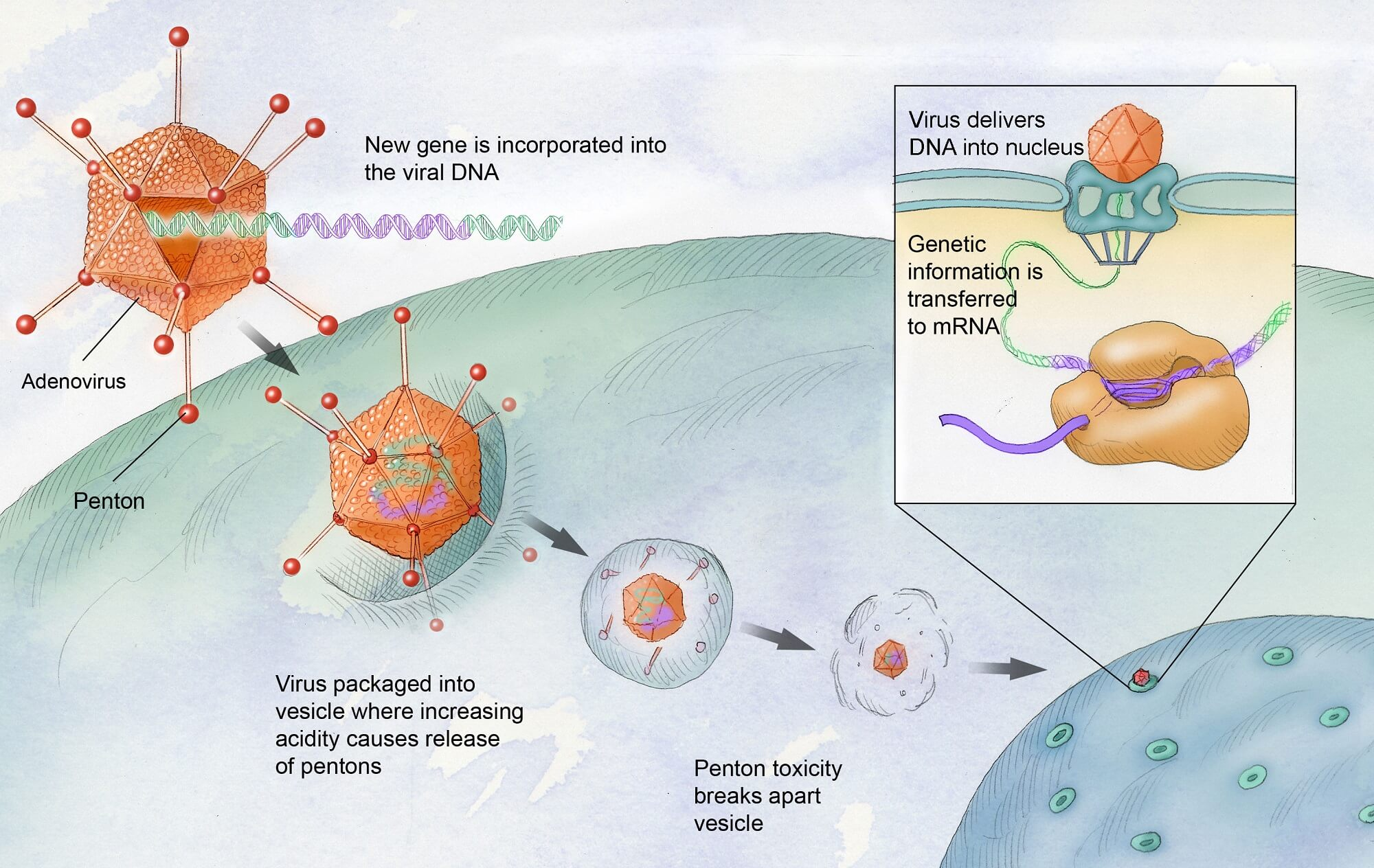
Gene therapy for hemophilia represents a monumental leap forward in medical science. Unlike traditional treatments that require ongoing administration of clotting factors, this innovative approach aims to correct the genetic defect at its source by delivering functional copies of the clotting factor gene directly to the patient’s cells. In February 2025, Terence Blue became the first patient in New England to receive Hemgenix, a groundbreaking gene therapy that holds the promise of reducing or eliminating the need for regular factor IX infusions. This therapy’s FDA approval signifies its potential to change the landscape of hemophilia treatment significantly.
The implications of gene therapy extend beyond mere symptom management. For patients like Blue, the prospect of a life free from daily injections is life-changing. With Hemgenix, the hope is not only to stop the bleeding but to enhance overall quality of life. As patients experience a newfound freedom and reduced anxiety over potential bleeding episodes, the success stories emerging from clinical trials spark optimism in both the medical community and among families affected by hemophilia. While gene therapy is not without its challenges, including high costs and varying levels of patient acceptance, its success stories underscore its transformative potential.
Benefits of Gene Therapy: Transforming Lives
The benefits of gene therapy extend beyond medical treatment; they encompass psychological and social improvements as well. For many individuals living with hemophilia, the constant burden of treatment and vigilance takes a toll on mental health and social interactions. The introduction of gene therapy into their treatment options can lead to a significant decrease in anxiety related to bleeding risk, allowing patients to engage more fully in daily activities and social situations without fear. Terence Blue’s journey reflects a narrative of hope—not just for fewer treatments but for reassurance in personal and social domains.
Moreover, the advancements in gene therapy technologies reflect robust research and the promise of innovation in medical sciences. Patients can expect not only prolonged efficacy from such treatments but also an improved safety profile. For many, the prospect of a near ‘cure’ seems within reach, with studies indicating that a substantial percentage of patients treated with Hemgenix continue to show no need for ongoing clotting factor therapy years post-treatment. This transformative impact of gene therapy could redefine the lives of hemophilia patients by offering pathways to health and normalcy previously unimagined.
Gene Therapy Success Stories: Inspiring Hope
As clinical trials for gene therapies continue to unfold, many success stories emerge, showcasing the tangible benefits for patients battling hemophilia. The case of Terence Blue is particularly inspiring as he represents a pivotal moment in a long journey toward effective treatment. His experience illustrates the potential of gene therapy not just to manage symptoms but to alter the course of his life dramatically. This wave of successful outcomes contributes to a growing database of real-world efficacy, where patients like Blue exhibit improved clotting factor levels and reduced reliance on traditional treatments.
The enthusiasm surrounding these success stories plays a crucial role in patient empowerment and decision-making. Potential patients and their families often look to these accounts for encouragement and insight as they navigate the complex landscape of hemophilia treatments. Knowing that others have undergone the therapy and experienced positive results fosters a sense of community and shared optimism. The narrative of gene therapy success continues to reshape expectations for hemophilia care, steering patients toward embracing innovative solutions and participating in their health journeys actively.
Market Pressures: Navigating Gene Therapy
While the promise of gene therapy for hemophilia is significant, the market dynamics often complicate the landscape for these therapies. The reality is that groundbreaking treatments come with hefty price tags and must navigate the intricate reimbursement process within healthcare systems. As discussed by experts like Roger Hajjar, it’s critical to find a balance between scientific innovation and the economic viability of using gene therapies. High prices may lead to diminished patient access, risking the advancement of research if the treatment landscape does not adjust to ensure patient need and affordability.
With past treatments being withdrawn due to insufficient market interest or high costs, the survival of new gene therapies is in question. Effective communication between researchers, healthcare providers, and patients is essential to ensuring that the innovation of gene therapy reaches those who need it most. Stakeholders must consider patient acceptance and provide adequate education regarding the long-term benefits and transformative potential of therapies like Hemgenix, ensuring that gene therapies not only survive but thrive in the competitive pharmaceutical landscape.
FDA Approved Gene Therapy: A New Standard
The approval of gene therapies by the FDA marks a notable milestone in the treatment of hemophilia, setting a new standard for addressing genetic disorders. With the advent of Hemgenix as an FDA-approved therapy in November 2022, the immediacy of change can be felt within the hemophilia community. Patients, caregivers, and healthcare professionals now have access to an alternative that holds the promise of long-term solutions, reflecting significant advancements in biotechnology and medical research.
This regulatory approval symbolizes the culmination of years of rigorous research and reflects the potential for widespread adoption of gene therapy as a first-line treatment for hemophilia. It also galvanizes further research into similar therapies for other genetic conditions, showcasing the FDA’s commitment to innovation in medicine. With the emergence of FDA-approved gene therapies, patients can begin to envision a future where debilitating conditions can be managed effectively, if not cured entirely, representing hope for many families affected by hemophilia.
Bridging the Gap: Research and Patient Care
The dialogue between research and patient care is vital in the advancement of gene therapy for hemophilia. As researchers push the boundaries of science, healthcare providers must integrate these innovations into clinical practice, ensuring that patients receive the best care possible. Researchers like Nathan Connell emphasize the importance of translating laboratory breakthroughs into meaningful treatments that genuinely impact patients’ lives, reinforcing the connection between scientific discovery and practical application.
Encouraging collaboration between academic institutions, pharmaceutical companies, and healthcare providers enhances communication about emerging treatments and their implications. As gene therapy continues to evolve, patient care models must adapt concurrently to leverage the potential benefits of these therapies fully. By bridging the gap between research advancements and clinical implementation, healthcare systems can cultivate an environment where innovative treatments not only exist in theory but find their way into the hands of patients who need them.
Living with Hemophilia: Coping and Thriving
Living with hemophilia includes both physical and emotional challenges that extend beyond medical treatment. Patients must navigate the realities of spontaneous bleeding, lifestyle adjustments, and social stigma—all while adhering to a rigorous treatment regimen. While gene therapy like Hemgenix shows promise in easing some of these burdens, the psychological impacts of living with a chronic condition remain profound. Terence Blue’s experience highlights the importance of finding a community and reliable support systems that empower individuals with hemophilia to not just cope but flourish.
Beyond medical advancements, fostering a supportive environment allows patients to share their experiences and foster resilience. For many, engaging in activities that promote physical well-being and social connection can lead to improved quality of life. Embracing therapeutic options, including counseling and support groups, goes a long way toward addressing the multifaceted nature of living with hemophilia. As patients like Blue pave the way for new perspectives on living with chronic conditions, the emphasis on mental health and community support becomes even more critical.
Future Directions: Gene Therapy’s Role in Hemophilia Care
The future of hemophilia care is poised for transformation as innovations like gene therapy take center stage. As more patients experience the benefits of therapies such as Hemgenix, researchers are encouraged to delve deeper into genetic solutions for other bleeding disorders and hereditary conditions. There is an optimistic outlook as the medical community witnesses the effectiveness of gene therapies in changing lives—transforming them from disease-focused to health-centered perspectives.
Ultimately, however, the challenge remains in ensuring equitable access to these therapies. As costs are driven by extensive research and development, balancing the economic implications with patient needs will be paramount. The emerging narrative surrounding gene therapy reflects a hopeful path forward, where personalized medicine can redefine treatment paradigms in hemophilia and beyond, promoting long-term health benefits while ensuring patients are at the heart of care.
Frequently Asked Questions
What is gene therapy for hemophilia and how does it work?
Gene therapy for hemophilia aims to correct the genetic mutations responsible for the disease, specifically hemophilia B, by introducing a normal copy of the gene responsible for producing clotting factor IX. This treatment typically involves using a modified virus to deliver the gene directly to the liver, where the clotting factor is produced, potentially reducing or eliminating the need for regular clotting factor injections.
What are the potential benefits of gene therapy for hemophilia treatment?
The benefits of gene therapy for hemophilia treatment include the possibility of long-lasting effects that may significantly reduce or eliminate the need for frequent injections of clotting factors. Patients like Terence Blue have reported faster healing times after injuries, improving their quality of life and reducing the daily burden of managing the condition.
Are there any FDA approved gene therapy options for hemophilia currently available?
Yes, Hemgenix is an FDA approved gene therapy for hemophilia B, which was granted approval in November 2022. It represents a significant advancement in hemophilia treatment, offering patients a potential one-time therapy that could reduce or negate the need for ongoing treatments.
What are some success stories of gene therapy for hemophilia?
Success stories of gene therapy for hemophilia include the case of Terence Blue, the first patient in New England to receive Hemgenix. After treatment, his factor IX levels improved significantly, allowing him to heal faster from injuries and reducing his reliance on traditional clotting factor treatments, demonstrating the transformative potential of gene therapy.
What are the challenges associated with gene therapy for hemophilia?
Challenges associated with gene therapy for hemophilia include high treatment costs, potential market pressures that could limit availability, and the need for further patient education and acceptance. Additionally, the effectiveness of treatments and their accessibility can vary, as seen with the withdrawal of some gene therapies from the market due to limited uptake.
How does gene therapy for hemophilia differ from traditional treatments?
Gene therapy for hemophilia differs from traditional treatments by aiming to provide a long-lasting solution in a single treatment, potentially correcting the underlying genetic cause of hemophilia. In contrast, traditional treatments involve regular injections of clotting factors to manage symptoms, which can be burdensome and time-consuming for patients.
What advancements have been made in gene therapy for hemophilia in recent years?
Recent advancements in gene therapy for hemophilia include the development and FDA approval of Hemgenix, new gene delivery methods using viruses, and promising clinical trial results showing long-term effectiveness in patients. Continued research is expanding the range of conditions targeted by gene therapies, offering hope for future treatments.
What are the long-term effects of gene therapy for hemophilia?
The long-term effects of gene therapy for hemophilia include the potential for sustained production of clotting factor IX, resulting in fewer bleeding episodes and less reliance on factor replacement therapies. Early data from clinical trials have shown that the majority of treated patients maintained adequate factor levels years after treatment.
| Key Point | Details |
|---|---|
| Terence Blue’s Experience | Terence Blue was the first patient in New England to receive gene therapy for hemophilia B at Brigham and Women’s Hospital in February 2025. |
| Advantages of Gene Therapy | Offers potential long-term relief from daily injections; boosts clotting factor IX production. |
| Market Challenges | High treatment costs ($3.5 million); market and patient acceptance issues leading to withdrawal of some therapies. |
| Clinical Outcomes | 94% of patients treated with Hemgenix did not require factor IX prophylaxis three years post-treatment. |
| Future of Gene Therapy | Ongoing research and optimism in developing new therapies; shift towards making gene therapy more applicable. |
Summary
Gene therapy for hemophilia is revolutionizing how patients manage their condition, exemplified by Terence Blue’s groundbreaking treatment experience. This innovative approach offers hope for long-term relief from the burden of daily injections and enhances the body’s ability to produce crucial clotting factors. However, while the potential for transformation exists, challenges such as high costs and market dynamics pose significant hurdles. As advancements continue, the future of gene therapy for hemophilia looks promising, potentially changing countless lives.