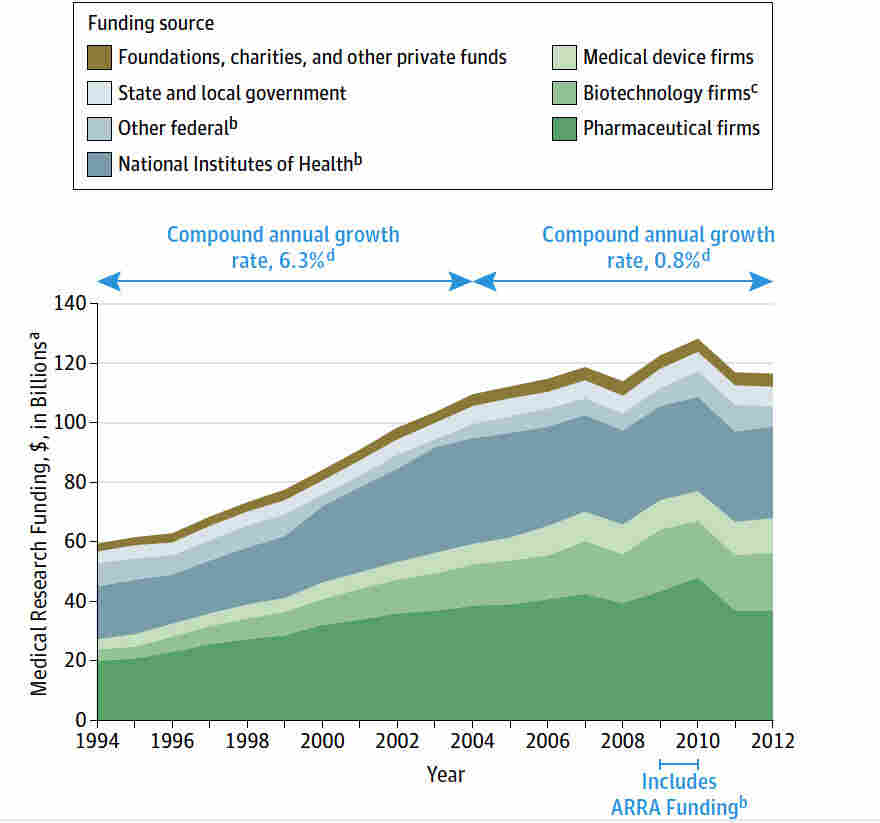
Medical research funding is crucial for advancing healthcare, yet recent funding cuts have posed significant challenges to the integrity of research designed to protect patient safety. The freeze on more than $2 billion in federal grants has disrupted important oversight mechanisms administered by institutional review boards (IRBs), jeopardizing the ethical conduct of clinical studies. As collaborative research becomes more complex, the impact of funding cuts is felt not just at the institutional level but also among the patients who depend on these studies for innovative treatments and therapies. Without adequate NIH research grants to support these initiatives, the very foundation of patient safety in research is threatened, leading to potential lapses in oversight and compliance. The ongoing challenges underscore the essential role of IRBs in maintaining ethical standards and ensuring that the rights and welfare of research participants are safeguarded, thereby highlighting the urgent need for adequate funding in medical research.
Funding for biomedical inquiry plays a pivotal role in the landscape of healthcare innovation. The recent disruptions in financial support have sparked concern about the ethical implications for participants involved in clinical studies, especially given the vital IRB oversight role. Such funding not only sustains research efforts but also enhances patient well-being and trust in the research process. As collaborative studies vie for financial resources, the increasing hurdles exacerbate collaborative research challenges and threaten the safety of patients enrolled in trials. Thus, ensuring consistent financial backing is imperative for fostering a research environment that prioritizes ethical standards and participant protection.
The Consequences of Medical Research Funding Cuts
The recent halt in medical research funding has sent shockwaves through institutions relying heavily on federal grants. In particular, the freeze of over $2 billion in federal research grants to Harvard has disrupted many critical projects, especially those focusing on patient safety in medical research. The implications of these funding cuts extend beyond just the immediate loss of resources; they fundamentally alter the operational capabilities of institutions that ensure the ethical treatment of study participants.
Without adequate funding, institutions face challenges in maintaining robust oversight systems essential for safeguarding patient safety. IRBs play a crucial role in this regard, providing thorough reviews of research proposals to assess risks and benefits. However, with the ongoing financial crunch, many projects might be forced to halt, causing delays in necessary research that could lead to advancements in medical therapies or devices, thereby impacting countless lives.
The Role of NIH Research Grants in Patient Protection
NIH research grants are pivotal in supporting studies that prioritize participant safety and ethical standards. By funding research that adheres to stringent IRB oversight, these grants ensure that various ethical considerations, such as informed consent and risk assessment, are at the forefront of clinical trials. This infrastructure is vital for building public trust in medical research and fostering ethical standards across institutions.
Moreover, NIH grants help facilitate multisite collaborations through mechanisms like the single IRB review, making it easier for research teams across different locations to work together while maintaining ethical oversight. This collaborative approach not only improves the efficiency of research but also enhances the safety protocols in place, ensuring that the rights and welfare of study participants are consistently prioritized.
Understanding the Impact of IRB Oversight on Medical Research
Institutional Review Boards (IRBs) are crucial in safeguarding patient interests, serving as the ethical backbone of medical research. They meticulously review each research proposal, focusing on protecting participant rights, evaluating risks versus benefits, and ensuring thorough informed consent processes are in place. This oversight becomes even more critical when collaborative research involves multiple sites, highlighting the importance of a streamlined review process, such as that implemented by NIH-funded projects.
However, reductions in funding could significantly impair the ability of IRBs to perform their duties effectively. Lower budgets may lead to fewer staff members and reduced capacity for training and education, which could compromise the quality of oversight. In the long run, this decline in regulatory rigor may lead to lapses in patient safety, diminishing public confidence in clinical trials and research outcomes.
Collaborative Research Challenges: The Need for Sustained Funding
Collaborative research among multiple institutions often brings about significant challenges, particularly in the context of funding cuts. When essential studies are halted or delayed due to financial restraints, it not only impacts the immediate research landscape but also hampers long-term innovation within the medical field. As federal grants shrink, institutions struggle to maintain collaborative efforts necessary for groundbreaking studies on urgent health issues.
This persistent lack of funding can create a ripple effect, where trust and communication between researchers and the communities they serve deteriorate. Stakeholders may become skeptical of research intentions and methodologies, negatively impacting participation rates in critical studies. Sustainable funding is essential to overcome these collaborative challenges, enabling researchers to embark on innovative projects that require teamwork across various disciplines and institutions.
The Importance of Ethics in Medical Research Practice
Ethical oversight in medical research is paramount, ensuring that participants are treated with dignity and respect. The historical context of medical research emphasizes the need for robust protective measures, as evidenced by past ethical breaches. Today, IRBs are central to enforcing these standards and ensuring compliance with regulations, particularly in studies involving vulnerable populations.
Without sustained oversight and adequate funding, the integrity of medical research practices can be compromised. The potential harms of neglecting to uphold ethical standards can undermine the trust between researchers and participants, leading to a reluctance to engage with clinical trials. Thus, it is critical to prioritize funding that supports ethical research practices, safeguarding both participants and the advancement of medical science.
Financial Implications of Medical Research on Health Outcomes
The financial implications of research funding go beyond just institutional budgets; they critically affect health outcomes across populations. Robust funding allows for thorough investigation into new treatments and technologies, ultimately leading to improved patient health. Conversely, when funding is cut or restricted, the progress made toward discovering innovative solutions is jeopardized, posing threats to public health.
Moreover, the cancellation of grants not only stalls individual studies but disrupts entire research ecosystems. Healthcare advancements that rely on collaborative efforts and diverse research teams risk stagnation in an environment of financial austerity. Hence, ensuring stable funding streams becomes essential for advancing medical knowledge and translating research findings into effective clinical practices that enhance patient well-being.
Navigating the Post-COVID Era of Medical Research Funding
As the world emerges from the COVID-19 pandemic, the landscape of medical research funding faces unprecedented challenges and opportunities. While the global health crisis highlighted the critical need for robust funding mechanisms, the subsequent cuts can hinder recovery efforts aimed at mitigating ongoing health issues exacerbated by the pandemic. Institutional and federal stakeholders must navigate these complexities to foster an environment conducive to innovation.
Future funding models should emphasize flexibility and adaptability in supporting diverse research initiatives that address the complexities of post-pandemic healthcare. This includes fostering collaborative frameworks among research institutions to optimize resource utilization and streamline oversight processes through agencies like the NIH. Sustained focus on research funding will be pivotal in enhancing patient safety and advancing medical knowledge in the years ahead.
Public Trust and Confidence in Research: The Role of Funding
Public trust in medical research is intricately linked to perceptions of the ethical oversight and funding allocated to such studies. When funding cuts occur, they not only compromise the operational capacities of research institutions but also raise concerns about the integrity of research outcomes. Participants are less likely to engage in studies if they feel their safety and rights are not adequately protected due to financial constraints.
Rebuilding public trust requires transparency in research processes and ensuring that adequate resources are directed toward ethical oversight mechanisms. Engaging communities in discussions about funding and oversight changes can foster a sense of participation and ownership in the research process. As trust is a cornerstone of successful clinical trials, strategic investments in ethical research practices are crucial for maintaining public confidence.
The Future of Medical Research: Collaborations and Funding Strategies
Looking toward the future of medical research, establishing effective collaborations will be key in overcoming challenges posed by funding cuts. By sharing resources and expertise across institutions, researchers can mitigate the negative impacts of financial limitations, thereby sustaining essential studies that enhance patient safety and well-being. Collaborative frameworks can also foster a culture of innovation, allowing for the exploration of new areas of inquiry.
Moreover, diversifying funding sources beyond traditional federal grants can enhance the resilience of research institutions. By exploring private partnerships, philanthropic donations, and public-private collaborations, the medical research community can create a more sustainable funding landscape. Through strategic alliances, researchers can ensure that critical studies continue, ultimately leading to advances that benefit society as a whole.
Frequently Asked Questions
How do funding cuts impact patient safety in medical research?
Funding cuts can severely affect patient safety in medical research by disrupting essential oversight mechanisms. With significant federal funding reductions like the recent cessation of $2 billion at Harvard, institutions are unable to maintain their Institutional Review Boards (IRBs) effectively. This results in delayed studies and inadequate monitoring of research practices, increasing the risk of harm to participants.
What is the role of NIH research grants in medical research funding?
NIH research grants play a crucial role in funding medical research, including vital safeguards for patient safety. These grants help cover the indirect costs associated with oversight, such as IRB reviews, ensuring compliance with ethical standards that protect research participants. Without adequate NIH funding, the integrity and safety of clinical studies are compromised.
Why is the IRB oversight role critical in medical research funding?
The IRB oversight role is essential in medical research funding because it ensures that all studies involving human participants comply with ethical standards. IRBs assess research proposals for ethical implications and safeguard participant rights throughout the study. Funding cuts that limit IRB functionality can undermine these protections, leading to potential safety concerns for participants.
What challenges arise in collaborative research due to cuts in medical research funding?
Collaborative research efforts often face significant challenges when medical research funding is cut. Funding reductions can hinder the ability to coordinate multiple sites effectively and can stall necessary collaborations. Institutions may be unable to participate in joint studies, creating barriers to innovation and delaying critical medical advancements.
How do funding cuts affect the ethics and oversight in medical research?
Funding cuts heavily impact the ethics and oversight of medical research by limiting the resources available for IRBs and other regulatory bodies. With fewer financial resources, these entities may struggle to uphold rigorous standards of oversight, leading to ethical lapses and potential risks for participant safety.
| Key Points |
|---|
| Impact of Federal Funding Cuts |
| The Trump administration’s freeze of over $2 billion in federal research at Harvard disrupts oversight and safety efforts in medical studies. |
| Role of IRBs |
| Institutional Review Boards (IRBs) are critical in ensuring the rights and welfare of research participants through oversight and ethical review of studies. |
| Historical Context |
| Past abuses in medical research have led to the establishment and evolution of ethical guidelines, demonstrating the need for continuous oversight of research practices. |
| Consequences of Research Halt |
| Halting studies midstream risks harm to participants and could erode public trust, making future research collaborations more difficult. |
| Continued Support from Harvard |
| Despite funding cuts, Harvard Medical School supports ongoing research efforts to maintain ethical standards and protect public health. |
Summary
Medical research funding is critical for ensuring the safety and rights of patients participating in clinical trials. The recent freeze of federal funding challenges the core structure that protects these patients and puts future research endeavors at risk. Without adequate funding, oversight systems like IRBs, which are designed to safeguard patient welfare, become vulnerable. This may lead to significant disruptions in ongoing studies and erode public trust in medical research, complicating efforts to advance scientific knowledge and therapeutic innovations. Ensuring robust funding for medical research is essential to maintain ethical standards and protect the health of all participants.