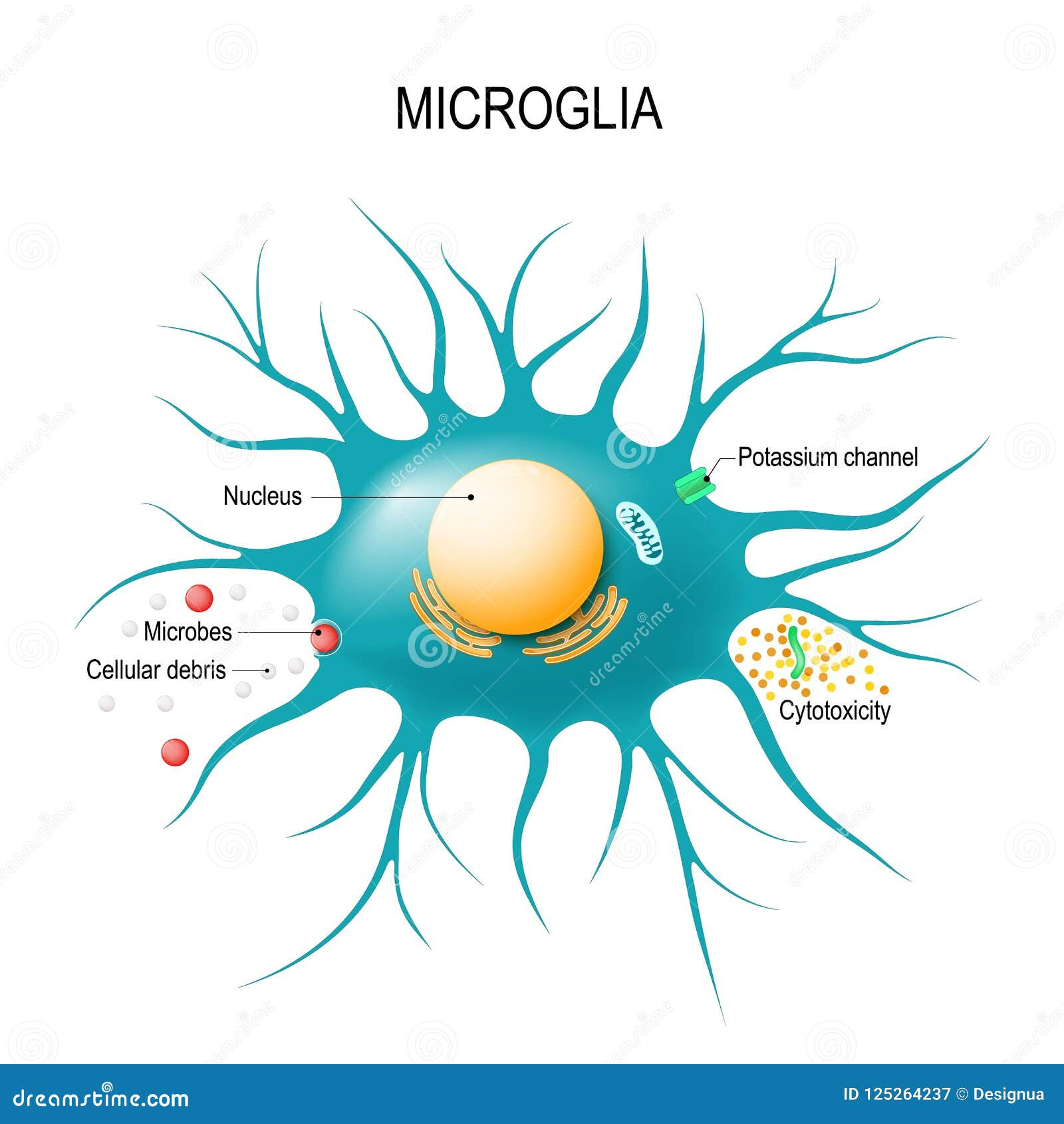
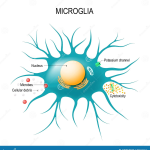
Microglial cells play a crucial role as the brain’s immune system, significantly influencing our understanding of neurodegenerative diseases such as Alzheimer’s. These specialized cells actively patrol the brain, seeking out damaged neurons and excess synapses to prune, a process essential for maintaining optimal brain function. As noted by prominent neuroscientist Beth Stevens, this delicate balance of synaptic pruning can sometimes go awry, leading to harmful consequences for brain health. Her groundbreaking research at the Stevens Lab has revealed how abnormal microglial activity may contribute to diseases like Alzheimer’s and Huntington’s, highlighting their importance in Alzheimer’s research. With an estimated 7 million Americans affected by Alzheimer’s, understanding the mechanisms behind microglial functions is key to developing new treatments and biomarkers, paving the way for advancements in the field of neurodegenerative disease.
The investigation of brain immune cells, often referred to as microglia, has revolutionized our perception of neurological health and disease. These remarkable cells act as guardians of the central nervous system, engaging in vital processes such as the clearance of neuronal debris and the refinement of neuronal circuitry through synaptic pruning. Pioneering studies, particularly those led by researchers like Beth Stevens, have underscored their pivotal involvement in the pathology of neurodegenerative disorders, including Alzheimer’s disease. By exploring how these immune cells behave and interact within the brain, scientists aim to unlock new therapeutic avenues that can reshape the landscape of brain health. This multi-faceted exploration into the brain’s immune environment not only enhances our grasp of neurodegeneration but also fuels innovations in treatment and prevention strategies.
The Role of Microglial Cells in Alzheimer’s Research
Microglial cells are becoming increasingly recognized for their pivotal role in the brain’s immune system, particularly in the context of Alzheimer’s research. These specialized cells patrol the central nervous system, identifying and removing debris from dead or unhealthy neurons. This process, known as synaptic pruning, is fundamental during the brain’s developmental stage and helps maintain neuronal networks. However, in neurodegenerative diseases like Alzheimer’s, this careful orchestration can go awry, leading to excessive pruning and the subsequent degeneration of neural circuits.
Beth Stevens and her team have illuminated the dual nature of microglial cells—they can act protectively but also contribute to the pathology of Alzheimer’s disease. Their groundbreaking findings highlight that aberrant microglial activity can escalate neuroinflammation and result in detrimental outcomes for synaptic health. By elucidating the mechanisms governing microglial function in Alzheimer’s pathology, Stevens’ research opens new avenues for potential treatments, signaling a paradigm shift in how we approach neurodegenerative diseases.
Synaptic Pruning and Neurodegenerative Diseases
Synaptic pruning is a crucial process for refining neuronal networks, essential for learning and memory. During normal brain development, microglial cells facilitate this pruning to enhance synaptic efficiency and connectivity. However, in the context of neurodegenerative diseases such as Alzheimer’s, Huntington’s disease, and others, this process can become dysregulated, leading to detrimental outcomes. Stevens’ research underscores how this mismanagement of synaptic pruning by microglial cells can contribute to the progression of these diseases, highlighting an intersection between immune function and synaptic health.
Understanding the abnormalities in synaptic pruning can reveal underlying mechanisms of neurodegeneration, offering insights for potential therapeutic approaches. By targeting microglial behavior, researchers can potentially mitigate harmful synaptic loss and promote healthier brain function. The ongoing investigation in this field is critical, as discovering biomarkers associated with dysfunctional pruning could lead to earlier interventions and improved care for millions affected by diseases like Alzheimer’s.
The Impact of Beth Stevens’ Research on Alzheimer’s Treatments
Beth Stevens’ pioneering work at the intersection of neuroscience and immunology has transformed our approach to Alzheimer’s disease. By uncovering the roles of microglial cells in synaptic pruning and their impact on neurodegenerative conditions, her research provides a framework for developing innovative therapies. The insights gained from her studies establish crucial links between immune responses in the brain and the mechanisms of Alzheimer’s pathology, emphasizing the importance of the brain’s immune system in maintaining cognitive health.
Moreover, this research underscores the significance of federal funding in advancing our understanding of complex diseases. Stevens highlights how support from organizations like the National Institutes of Health enabled her to conduct fundamental research that may one day lead to significant breakthroughs in treatment options. As we look to the future, Stevens’ contributions could pave the way for novel strategies that not only target Alzheimer’s symptoms but also address underlying causes and promote better long-term outcomes for patients.
Promising Biomarkers in Alzheimer’s Disease Research
Discovering effective biomarkers is a critical step in Alzheimer’s research, as they can facilitate early diagnosis and track disease progression. The work of Beth Stevens has brought to light the potential of biomarkers linked to microglial activity and synaptic health. By analyzing the interaction between microglial cells and synapses, researchers can identify specific markers that indicate abnormal pruning activity—a hallmark of neurodegenerative diseases. These insights could revolutionize both diagnostic approaches and treatment modalities in Alzheimer’s.
The identification of reliable biomarkers enables clinicians to detect Alzheimer’s disease at earlier stages, improving the chances of successful intervention. Furthermore, as recent findings suggest that some biomarkers might be modifiable through targeted therapies, there is hope that we can not only monitor the disease but also reverse some of its effects. Stevens’ work stands at the forefront of this initiative, leading to the development of diagnostic tools and treatment options that could significantly enhance patient care.
The Journey of Basic Science to Translational Research
The path from basic science to practical applications in Alzheimer’s therapy is often long and complex, as demonstrated by Beth Stevens’ journey in her research on microglial cells. Initially focused on understanding how these immune cells interact with neural networks, her work has gradually unraveled intricate mechanisms that connect normal brain function to neurodegenerative disease processes. This foundational research is crucial; it lays the groundwork for discovering new treatment interventions and technologies.
Stevens emphasizes that curiosity-driven research often leads to unexpected findings that significantly impact disease understanding. The exploration of microglial functions, for instance, has illuminated new pathways for therapeutic intervention in Alzheimer’s and other neurodegenerative diseases. As science continues to evolve, the continual support for basic research is vital, reinforcing the philosophy that every discovery builds toward a future where effective treatments are realized for patients suffering from debilitating conditions.
The Federal Funding Landscape for Alzheimer’s Research
The trajectory of Alzheimer’s research significantly relies on federal funding, which plays an essential role in supporting scientists like Beth Stevens. With grants from the National Institutes of Health, researchers can explore innovative ideas and conduct vital experiments that may lead to breakthroughs in understanding neurodegenerative diseases. This funding landscape is not just a financial support system; it cultivates an environment of exploration and discovery that drives the scientific community forward.
Stevens’ reflection on her dependence on federal funding highlights the necessity of sustained investment in basic science. It is through such financial backing that researchers can tackle complex questions regarding the brain’s immune system and its involvement in diseases like Alzheimer’s. As advocacy for funding continues, it is important to recognize the impact this support has on advancing our knowledge and developing effective treatments for millions at risk for or suffering from these conditions.
Exploring the Connection Between Neuroinflammation and Alzheimer’s
Neuroinflammation is a critical factor in the progression of Alzheimer’s disease, and microglial cells are at its helm. Research led by Beth Stevens emphasizes that while microglia are essential for brain health, their chronic activation can signal trouble. Under pathological conditions, these cells can exacerbate inflammation, which subsequently damages neurons and contributes to cognitive decline. By understanding these complex interactions, we can improve therapeutic strategies to mitigate neuroinflammation and slow Alzheimer’s progression.
Furthermore, unraveling the relationship between neuroinflammation and synaptic pruning reveals profound implications for treatment strategies. Strategies targeting microglial activation could potentially restore healthy synaptic pruning patterns, providing new hope for patients with Alzheimer’s disease. Thus, Stevens’ work not only enhances our understanding of neuroinflammatory processes but also sets the stage for novel interventions that may help to alleviate the burden of Alzheimer’s.
Transforming Neurodegenerative Disease Paradigms
The research conducted by Beth Stevens is transforming prevailing paradigms in neurodegenerative disease understanding. By highlighting the dual roles of microglial cells as both protectors and potential aggressors in the brain, her work urges a reconsideration of how we approach Alzheimer’s disease. Instead of merely focusing on neuronal loss and brain atrophy, her findings compel researchers to explore the dynamic interactions between immune cells and neurons, revealing a more complex picture of neurodegeneration.
This shift in perspective fosters a more holistic approach to Alzheimer’s research and treatment. As we recognize the importance of the brain’s immune system in orchestrating neural health, we can devise multifaceted strategies that not only target symptomatic relief but also address underlying immune dysregulation. The implications of Stevens’ research highlight the necessity for interdisciplinary collaborations to tackle the multifactorial aspects of Alzheimer’s and other neurodegenerative diseases.
Future Directions in Microglial Research and Alzheimer’s Care
As we look to the future, microglial research continues to be a frontier in Alzheimer’s disease studies. Emerging technologies and methodologies are providing unprecedented insights into microglial behavior under both normal and pathological conditions. Beth Stevens’ groundwork lays a solid foundation for further exploration into how these immune cells can be manipulated to enhance brain health and combat neurodegenerative diseases. The potential for discovering new therapeutic targets is vast.
Moreover, integrating findings from microglial research into clinical practices will be essential for translating scientific discoveries into tangible benefits for patients. The ongoing collaboration between basic researchers and clinicians will ensure that revolutionary findings in the lab can inform innovative treatments in the clinic. As Stevens advocates, continued investment in this line of research holds promise for transforming Alzheimer’s care and improving the lives of millions affected by neurodegenerative diseases.
Frequently Asked Questions
What role do microglial cells play in Alzheimer’s research?
Microglial cells serve as the brain’s immune system and are crucial in Alzheimer’s research. They help remove dead or damaged cells and are involved in synaptic pruning, which is the process of eliminating unnecessary synapses. Aberrant pruning by microglia has been implicated in the progression of Alzheimer’s disease, making them a significant focus for researchers like Beth Stevens in developing potential treatments.
How do microglial cells contribute to neurodegenerative diseases?
Microglial cells contribute to neurodegenerative diseases by responding to brain injuries or diseases. In conditions like Alzheimer’s and Huntington’s diseases, abnormal activation of microglia can lead to excessive synaptic pruning, which may worsen neuronal degeneration. Understanding their role is vital for developing new biomarkers and therapies.
Who is Beth Stevens and what is her significance in the study of microglial cells?
Beth Stevens is a leading neuroscientist whose research on microglial cells has transformed our understanding of their roles in the brain’s immune response and their impact on neurodegenerative diseases, particularly Alzheimer’s disease. Her work has highlighted how microglial dysfunction can lead to aberrant synaptic pruning, paving the way for new therapeutic strategies.
What is the connection between microglial cells and synaptic pruning?
Microglial cells are essential for synaptic pruning, a process that helps shape and refine neural connections during brain development. In healthy brains, microglia remove excess synapses that are not necessary for efficient communication between neurons. However, in neurodegenerative diseases like Alzheimer’s, this pruning process can become dysregulated, leading to increased neuronal loss.
How can understanding microglial cells lead to better treatments for Alzheimer’s disease?
Understanding microglial cells can lead to better treatments for Alzheimer’s disease by identifying new biomarkers for early detection and potential therapeutic targets. Research into how these cells function in the brain’s immune system and their role in synaptic pruning can inform strategies to prevent or reverse their abnormal activity, thus improving outcomes for patients.
What advancements have been made in Alzheimer’s research regarding microglial cells?
Recent advancements in Alzheimer’s research regarding microglial cells include identifying their key role in the immune response and synaptic pruning. Studies led by researchers like Beth Stevens have shown how microglial dysfunction can contribute to neurodegeneration. This knowledge is crucial for developing innovative diagnostic tools and therapeutic approaches to manage Alzheimer’s disease.
What challenges do researchers face when studying microglial cells and neurodegenerative diseases?
Researchers studying microglial cells and neurodegenerative diseases face challenges such as the complex interactions between microglia and other brain cells, the difficulties in replicating human brain conditions in animal models, and the need for long-term studies to understand the disease mechanisms. These challenges require ongoing funding and innovative approaches to fully unlock the potential of microglial research.
What potential do microglial cells hold for developing new biomarkers in Alzheimer’s disease?
Microglial cells hold significant potential for developing new biomarkers in Alzheimer’s disease by serving as indicators of neuroinflammatory changes and synaptic health. Researchers are exploring how microglial activation states correlate with Alzheimer’s progression, which could lead to novel diagnostic tools that allow for earlier intervention and personalized treatment strategies.
| Key Point | Details |
|---|---|
| Role of Microglial Cells | Microglial cells serve as the brain’s immune system, monitoring for illness or injury. |
| Pruning Function | They help clear dead or damaged cells and prune synapses, essential for neural communication. |
| Aberrant Pruning | Improper pruning by microglia may contribute to conditions like Alzheimer’s and Huntington’s diseases. |
| Research Significance | The findings from Stevens Lab have led to new biomarkers and potential treatments for neurodegenerative diseases. |
| Funding and Support | Stevens emphasized the importance of NIH funding in advancing research on microglial cells. |
| Curiosity-Driven Science | Basic science provides the questions and groundwork that lead to significant medical advancements. |
Summary
Microglial cells play a crucial role in maintaining brain health and combating neurodegenerative diseases like Alzheimer’s. Research conducted by Beth Stevens has transformed our understanding of these immune cells, revealing how they not only protect but can also harm the neuronal landscape when their pruning processes go awry. As we continue to explore the complexities of microglial functions, we pave the way for developing innovative therapies aimed at improving care for millions affected by debilitating brain disorders.