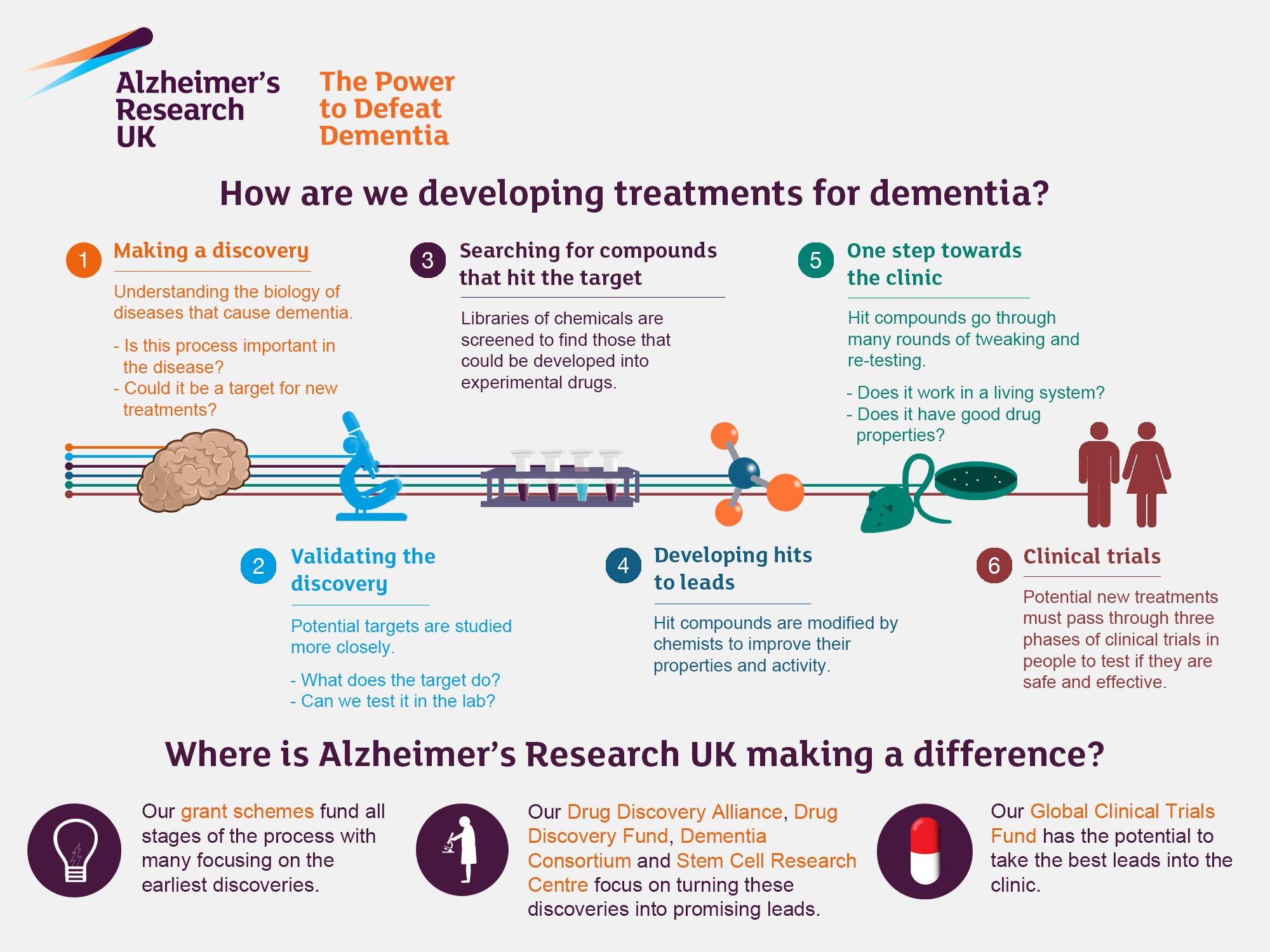
Alzheimer’s research is at the forefront of advancing our understanding of one of the most challenging neurodegenerative diseases facing the aging population today. Scientists, such as Beth Stevens, are exploring the role of microglial cells—key players in the brain’s immune system—that are crucial in maintaining neural health. These cells are responsible for clearing out damaged neurons and maintaining synaptic connections, but their faulty functioning may lead to the progression of Alzheimer’s disease. Stevens’ groundbreaking studies not only shed light on how aberrant pruning of synapses can exacerbate this condition but also pave the way for innovative Alzheimer’s treatments. With around 7 million Americans living with the disease, the urgency of this research cannot be understated, especially as the number of cases is projected to significantly increase in the coming decades.
The exploration of Alzheimer’s disease, a complex form of dementia, is becoming increasingly vital as our population ages. Neuroscientists like Beth Stevens are making strides in understanding how immune responses in the brain, particularly through microglial cells, can influence the development and progression of this debilitating condition. These cells form an integral part of the brain’s defense mechanism, tasked with eliminating toxic elements; however, their dysfunction may play a pivotal role in the onset of neurodegenerative disorders like Alzheimer’s. By comprehensively studying these immune responses, researchers are hopeful to discover new biomarkers for early detection and novel treatments that could potentially reverse or slow down the effects of the disease. As we continue to battle the challenges posed by age-related cognitive decline, the importance of Alzheimer’s research remains more relevant than ever.
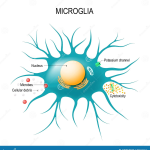
Understanding Microglial Cells: The Brain’s Immune System
Microglial cells play a crucial role in maintaining brain health by acting as the first line of defense in the brain’s immune system. These cells are responsible for monitoring the central nervous system, detecting and responding to injury, and clearing away dead or damaged neurons. The actions of microglia can influence neurological function significantly, and any dysregulation in their activity can lead to serious neurodegenerative diseases, including Alzheimer’s. This highlights the importance of ongoing research to fully understand microglial functions and their intricate interplay with other neural cells.
Recent findings, particularly those from Beth Stevens’ lab, indicate that microglial cells are also involved in synaptic pruning, a process that, while necessary for normal brain development, can result in harmful consequences if dysregulated. Aberrant pruning by microglia may contribute to the pathogenesis of neurodegenerative conditions such as Alzheimer’s disease. Therefore, studying these cells offers vital insights into potential therapeutic targets for treating Alzheimer’s and similar disorders, emphasizing the pivotal role of microglial research in advancing our understanding of brain health.
Alzheimer’s Research: A Transformative Approach to Neurodegenerative Diseases
Alzheimer’s disease represents one of the most significant challenges in contemporary neuroscience, necessitating innovative research approaches. Current understandings of the illness have been transformed by studies focusing on microglial cells, revealing how these immune cells contribute to neural degeneration. The research led by Beth Stevens not only contributes to our comprehension of Alzheimer’s but also provides pathways for identifying new biomarkers that could facilitate earlier diagnosis of neurodegenerative diseases, potentially transforming patient outcomes.
The implications of Stevens’ work extend beyond just Alzheimer’s disease; they also address other neurodegenerative conditions like Huntington’s disease. By dissecting the role of microglial cells in neural pruning and inflammation, researchers can develop targeted Alzheimer’s treatments that may halt or prevent the disease’s progression. As understanding builds around these complex cellular interactions, the hope remains that medical advancements can significantly improve the quality of life for millions affected by Alzheimer’s and similar conditions.
The Future of Alzheimer’s Treatments: Insights from Basic Science Research
The future of effective Alzheimer’s treatments hinges on the insights gained from foundational scientific research. As Beth Stevens has indicated, understanding the mechanisms at play in microglial function will be crucial for innovating therapies aimed at altering the course of Alzheimer’s disease. Relying on substantial support from agencies like the National Institutes of Health (NIH), researchers are building on basic principles that could lead to breakthroughs in how we approach treatment strategies and disease management.
One promising avenue of research is the exploration of how to modulate microglial activity to enhance their protective functions. Targeted therapies could be developed to prevent the harmful pruning of synapses while promoting the clean-up of neurotoxic elements. As more findings emerge from related studies, the hope is that a clearer pathway will be established for developing Alzheimer’s treatments that could not only slow disease progression but also improve neurodegenerative disease management overall.
The Role of Curiosity in Alzheimer’s Research
Curiosity-driven research plays a pivotal role in advancing our understanding of Alzheimer’s and other neurodegenerative diseases. Beth Stevens emphasizes that the foundational questions posed during early investigation into microglial cells paved the way for significant discoveries that might have initially seemed distant from practical application. This spirit of inquiry fuels innovative scientific ideas, leading to unexpected insights into complex biological processes that contribute to disease.
Recognizing the importance of curiosity extends beyond individual research efforts; it highlights the need for collaboration and shared knowledge within the scientific community. When researchers pursue their natural curiosities, they often uncover pathways that lead to breakthroughs in understanding disease mechanisms, ultimately shaping the future of Alzheimer’s studies. Encouraging such exploratory research is crucial for uncovering the complexities of neurodegenerative diseases, which in turn could lead to effective treatments.
Funding the Fight Against Alzheimer’s Disease
Funding plays a crucial role in the advancement of Alzheimer’s research. With the alarming projections for the increase in Alzheimer’s cases over the coming decades, supporting scientific investigations into the underlying causes and potential treatments is more important than ever. Stevens’ lab exemplifies how foundational research, supported by resources from federal grants, can lead to groundbreaking discoveries in the field of neurodegenerative diseases. This investment in science is essential for developing new Alzheimer’s treatments and improving care for affected individuals.
Increased funding enables researchers to explore innovative therapeutic strategies that target underlying mechanisms of disease, including microglial dysfunction and aberrant synaptic pruning. Such resources facilitate the development of comprehensive studies that not only investigate Alzheimer’s disease but also extend to other neurodegenerative disorders. As awareness grows about the need for continued investment in Alzheimer’s research, the hope is that more effective treatments can be discovered, ultimately leading to improved patient outcomes.
The Neuroscientific Importance of Beth Stevens’ Research
Beth Stevens’ contributions to neuroscience have initiated a paradigm shift in our understanding of microglial cells and their role in neurodegenerative diseases like Alzheimer’s. Her investigations highlight how these immune cells not only respond to neuronal damage but also engage in processes that could inadvertently lead to synaptic loss and neurodegeneration. This insight has significant implications for both basic scientific inquiry and the development of new therapeutic interventions.
By focusing on the intricate balance that microglia maintain in brain health, Stevens’ work encourages a multifaceted approach to studying Alzheimer’s disease. While previous research may have overlooked the immune system’s role in neurodegeneration, her findings underscore the necessity of an integrated perspective that combines immunology, neuroscience, and therapeutic development. This comprehensive understanding paves the way for future research efforts aimed at unraveling the complex etiology of Alzheimer’s.
The Impact of Aging on Alzheimer’s Disease Prevalence
As the population ages, the prevalence of Alzheimer’s disease is expected to rise dramatically, with projections estimating a doubling of annual cases by 2050. This increase correlates with not only demographic changes but also highlights the pressing need for continued research into the pathophysiology of aging and neurodegeneration. Investigations into the roles of microglial cells in aged brains are particularly important, as they may reveal how aging influences immune responses within the central nervous system.
Understanding the intersection of aging and Alzheimer’s disease is crucial for developing targeted interventions. As researchers like Beth Stevens uncover the mechanisms by which microglial activity alters with age, it becomes possible to identify new strategies for preventing or delaying the onset of Alzheimer’s in older adults. Addressing the challenges posed by an aging population remains a priority in the fight against this devastating disease.
Biomarkers in Alzheimer’s Disease Detection and Progression
The search for reliable biomarkers in Alzheimer’s disease is a key area of ongoing research that can revolutionize early diagnosis and monitoring of the disease’s progression. As Stevens’ research suggests, understanding microglial behavior could lead to the identification of markers that indicate aberrant cellular functions prior to the onset of clinical symptoms. Establishing such biomarkers is essential for timely intervention which can potentially delay or mitigate the decline associated with Alzheimer’s.
By linking specific microglial responses to particular stages or types of Alzheimer’s pathology, researchers can develop more precise diagnostic tools. This work not only aids in early detection but also allows for better tracking of disease progression and therapeutic effectiveness. Enhancing our ability to monitor Alzheimer’s through biomarker research creates new opportunities for personalized medicine, where treatments can be tailored to individual patients based on their unique disease profiles.
Innovative Approaches to Neurodegenerative Disease Research
Innovation is key to making significant strides in neurodegenerative disease research, particularly as we deepen our understanding of complex conditions like Alzheimer’s. Researchers are now employing cutting-edge technologies, including advanced imaging techniques and genetic analysis, to explore the role of microglial cells and other factors contributing to neural degeneration. These innovative tools are essential for gaining insights that can transform research findings into actionable treatments.
The integration of diverse scientific approaches is vital for addressing the multifactorial nature of Alzheimer’s disease. By combining knowledge from various fields, such as genetics, immunology, and neurology, researchers can formulate comprehensive strategies for tackling neurodegenerative diseases. This collaborative spirit, exemplified in the work of Stevens and her team, demonstrates the potential of interdisciplinary efforts in changing the landscape of Alzheimer’s research and treatment.
Frequently Asked Questions
What role do microglial cells play in Alzheimer’s research?
Microglial cells are critical in Alzheimer’s research as they act as the brain’s immune system, patrolling for signs of illness or injury. In the context of Alzheimer’s disease, these cells help clear out dead or damaged neurons and prune synapses. However, aberrant microglial activity has been linked to neurodegenerative diseases, suggesting that their function could provide insights for developing Alzheimer’s treatments.
How are microglia connected to neurodegenerative disease progression?
Microglia are central to the progression of neurodegenerative diseases, including Alzheimer’s. Recent studies have shown that improper pruning of synapses by microglial cells can contribute to disease pathology. Understanding this connection helps researchers identify potential therapeutic targets to slow down or halt the progression of Alzheimer’s disease.
What are the latest advancements in Alzheimer’s treatments based on microglial research?
The latest advancements in Alzheimer’s treatments involve targeting the dysfunction of microglial cells. Researchers, including Beth Stevens and her team, are investigating how to manipulate microglial activity to improve synaptic health and remove toxic debris more effectively. These findings may lead to novel therapeutic strategies for Alzheimer’s disease.
Who is Beth Stevens and what is her contribution to Alzheimer’s research?
Beth Stevens is a neuroscientist known for her pioneering work on microglial cells and their role in brain health. Based at Boston Children’s Hospital, her research has transformed our understanding of how microglia interact with neurons and their involvement in neurodegenerative diseases like Alzheimer’s. Stevens’ insights have laid the groundwork for new treatments and diagnostic methods.
What impact do microglial abnormalities have on Alzheimer’s disease?
Abnormalities in microglial function can exacerbate Alzheimer’s disease by leading to excessive synaptic pruning and inadequate clearance of toxic substances in the brain. This dysregulation contributes to neuroinflammation and neuronal death, which are hallmarks of Alzheimer’s pathology. Research in this area sheds light on potential interventions to restore normal microglial function.
Why is fundamental research important in the fight against Alzheimer’s disease?
Fundamental research, such as the studies conducted by Beth Stevens on microglial cells, is crucial for uncovering the underlying mechanisms of Alzheimer’s disease. This basic science forms the foundation for developing innovative treatments and biomarkers that can detect Alzheimer’s earlier, ultimately enhancing patient outcomes.
What are the implications of aging on Alzheimer’s research?
As the population ages, the incidence of Alzheimer’s disease is expected to rise significantly. This demographic shift highlights the urgency of Alzheimer’s research, particularly in understanding how microglial function changes with age. Insights from this research can help devise strategies to mitigate the risk of developing Alzheimer’s in older adults.
How can microglial research lead to earlier detection of Alzheimer’s disease?
Microglial research is paving the way for identifying new biomarkers that could enable earlier detection of Alzheimer’s disease. By understanding the role of microglial cells in synaptic health and their changes in Alzheimer’s pathology, researchers aim to develop diagnostic indicators that reveal the disease’s presence before significant neurodegeneration occurs.
| Key Point | Details |
|---|---|
| Neuroscientist Contribution | Beth Stevens focuses on microglial cells as part of the brain’s immune system. |
| Role of Microglia | Microglia clear damaged cells and prune synapses, which can affect brain health. |
| Impact of Findings | Research indicates aberrant pruning contributes to Alzheimer’s and other neurodegenerative diseases. |
| Potential of Research | Findings could lead to new medicines and biomarkers for early detection of Alzheimer’s. |
| Future Outlook | With the aging population, Alzheimer’s cases may double by 2050, straining healthcare costs. |
| Research Support | Stevens’s research has been significantly supported by NIH and federal funding. |
Summary
Alzheimer’s research is crucial in understanding and combating this devastating disease. The work by researchers like Beth Stevens, who explores the role of microglial cells in brain health, showcases a transformative approach that could redefine treatment strategies. Her pioneering findings highlight the importance of basic science in uncovering the intricacies of neurodegenerative conditions, paving the way for innovative treatments. As the prevalence of Alzheimer’s is projected to significantly increase, the continued investment in research and the exploration of new therapeutic avenues is essential for improving lives and managing future healthcare challenges.